In IgA Nephropathy
Elevated Proteinuria Increases Risk of Disease Progression
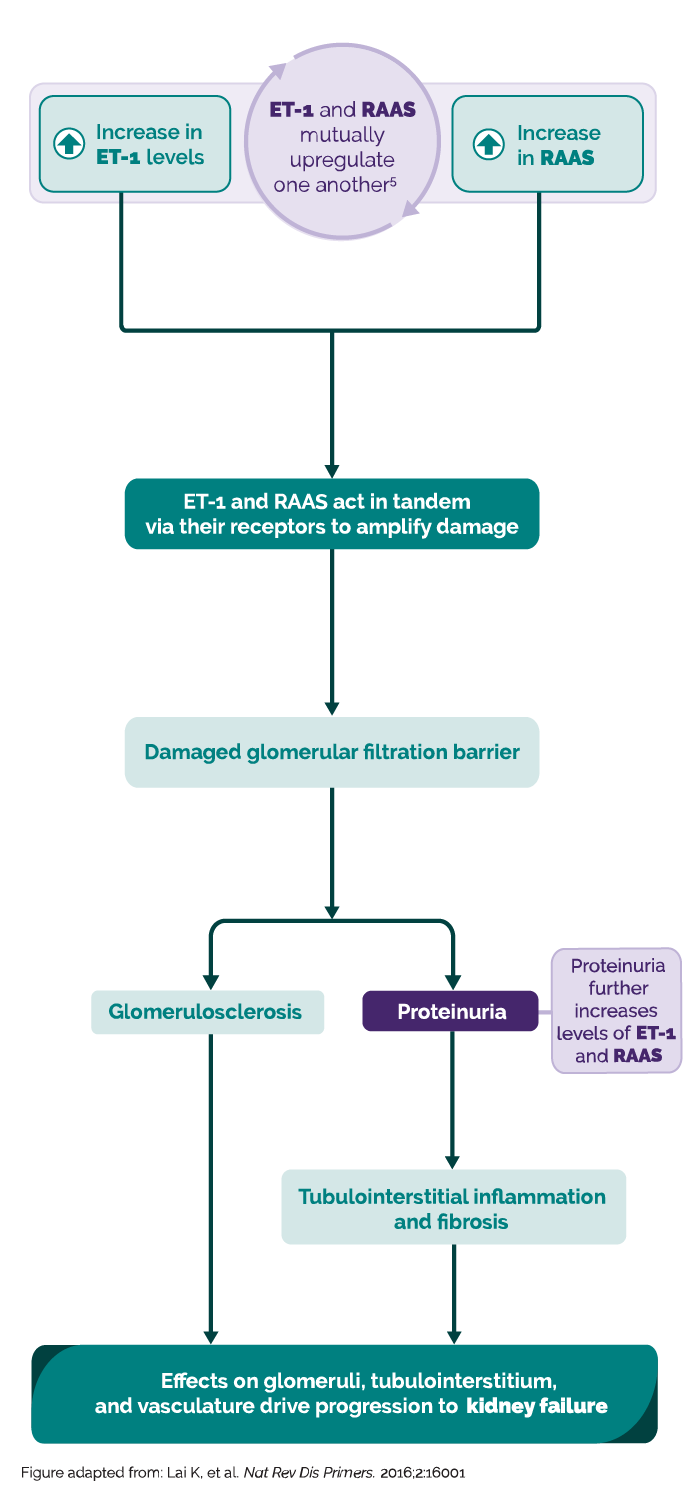
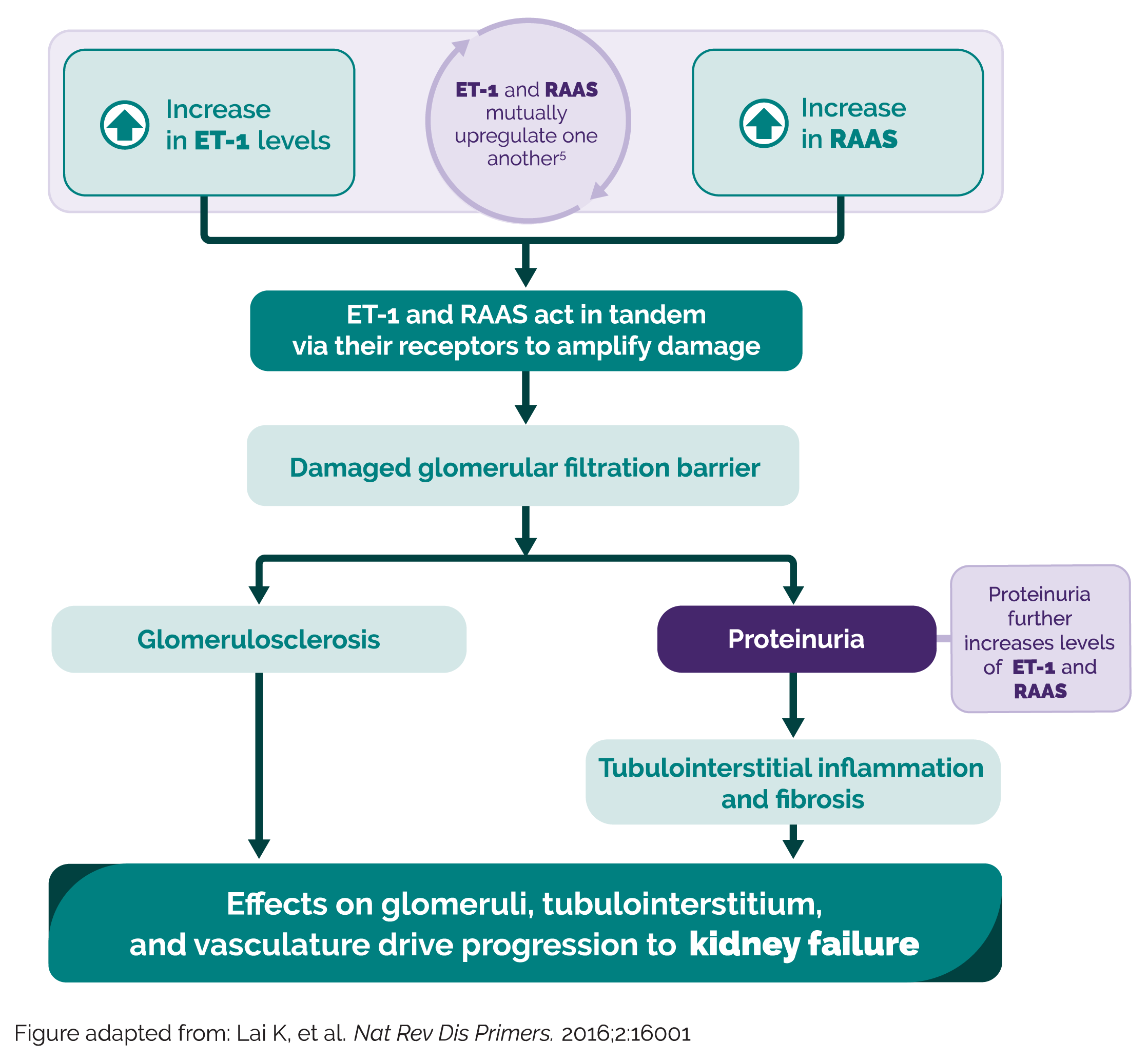
IgA nephropathy is driven by two key signaling pathways, Endothelin-1 (ET-1) and Renin Angiotensin Aldosterone System (RAAS)1-7
Take a closer look in this short video at how ET-1 and RAAS mutually upregulate one another to drive kidney disease progression
The hallmark of IgA nephropathy pathophysiology is the deposition of galactose-deficient IgA containing immune complexes in the mesangium1-7
Persistent proteinuria is the single strongest modifiable prognostic indicator for disease progression in IgA nephropathy8
New data from UK RaDaR analysis provide new insights into disease progression and proteinuria9
Retrospective cohort study – enrollment began in 2013

Patients progressing to kidney failure within 10 years of diagnosisa,b
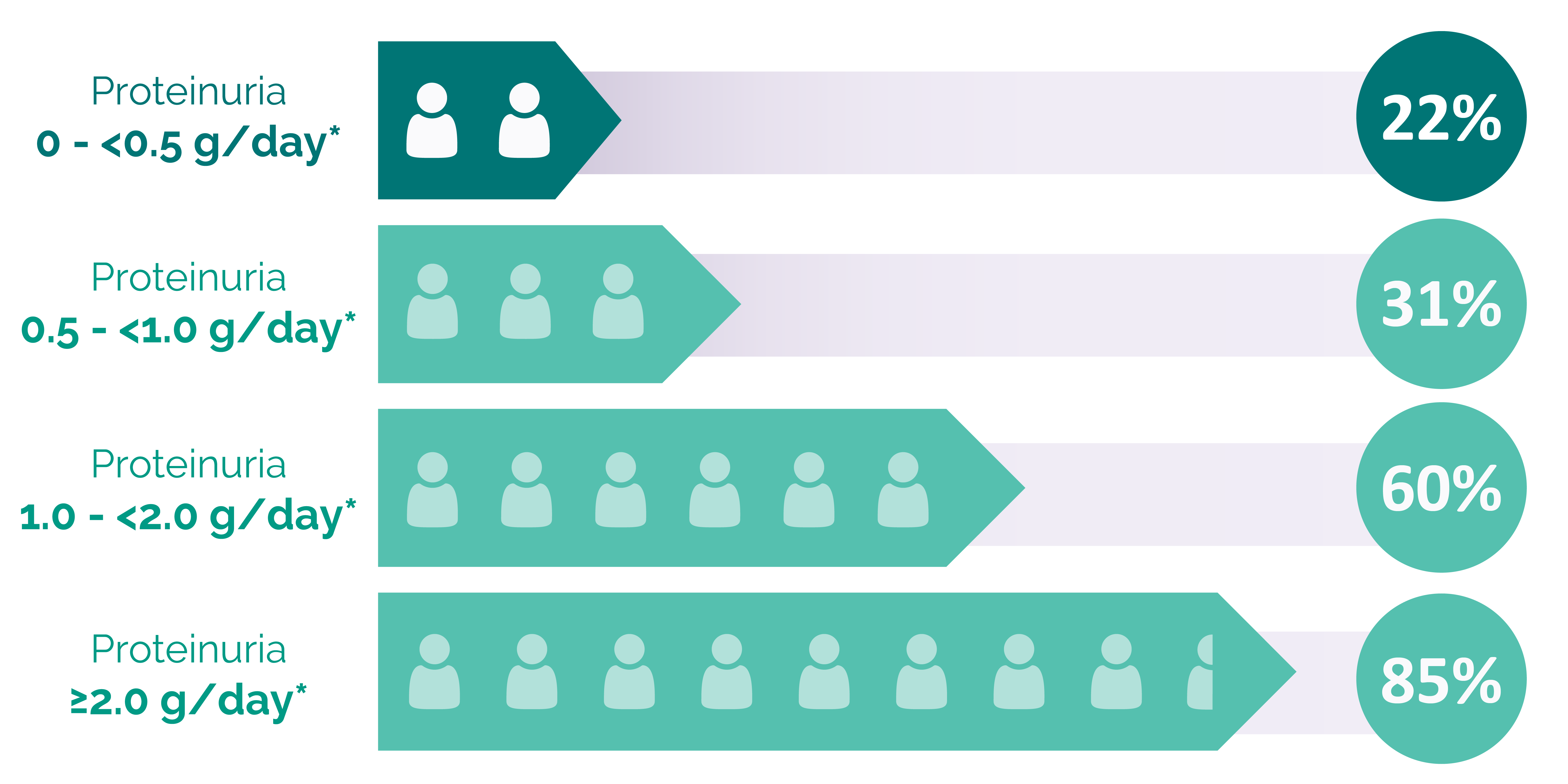
*UPCR 0.88 g/g ≈ 1 g/day
This analysis received funding from Travere Therapeutics. All instances of proteinuria refer to time-averaged proteinuria, defined as the time-weighted averages for UPCR. aThe adult patient subpopulation assessed is representative of an incident population, examining time-averaged proteinuria over follow-up without requirement for a baseline UPCR at diagnosis. bKidney failure defined as the first occurrence of either long-term kidney replacement therapy, a confirmed eGFR <15 mL/min/1.73 m2, or CKD stage 5.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UK, United Kingdom; UPCR, urine protein-to-creatinine ratio.
Many patients at proteinuria levels typically perceived as low risk had poor long-term outcomes9
RaDaR, UK National Registry of Rare Kidney Diseases.
Treatment options historically have been limited.
Kidney Disease: Improving Global Outcomes (KDIGO) guidelines 2021 recommend10:
- Renin-Angiotensin System (RAS) Inhibitors:
- Maximally tolerated dose of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) in all patients with proteinuria >0.5 g/day
- Glucocorticoids:
- 6-month course for patients at high risk of progressive CKD
KDIGO offers a “weak recommendation” of steroids in patients who remain at high risk of CKD progression, despite maximal supportive care, due to significant risk of toxicity.*
*KDIGO defines “weak recommendation” as “different choices will be appropriate for different patients,” and clinicians should help the patient arrive at a treatment decision.
KDIGO guidelines are expected to be updated at the end of 2023 or in early 2024; they are anticipated to include new treatment options.
The IgA nephropathy treatment landscape is quickly changing. Learn about an FDA-approved treatment option for reducing proteinuria in adult patients with IgA nephropathy.
Accurate prognosis leads to optimized patient care
The International Risk-Prediction Tool in IgA Nephropathy*
This simple-to-use tool, based on a risk-prediction model, was developed to assess long-term prognosis in patients with IgA nephropathy11-13:
- An at-biopsy version of the tool was designed for use at the time of biopsy-confirmed diagnosis11
- A post-biopsy version was designed for reassessment of risk at 1 to 2 years after biopsy13
- The tool predicts long-term risk of worsening kidney function, measured as a 50% reduction in eGFR or the development of end-stage kidney disease (ESKD)11,13
- The tool can be run with or without controls for ethnicity (Caucasian, Chinese, or Japanese)11-13
- The tool cannot be used to determine the likely impact of any particular treatment regimen10
- The tool incorporates clinical information at the time of biopsy and additional research is needed to determine if the output can be used at other time points in the progression of the disease11
2 ways to access the tool
Download the app:
Calculate by QxMD Mobile App
for iOS and Android devices
Go to the website:
Calculate by QxMD Website
for desktop and mobile browsers
*The International Risk-Prediction Tool in IgA Nephropathy was created by QxMD and is not a Travere Therapeutics sponsored tool.
Applying research to clinical practice in FSGS
ACEi, angiotensin-converting enzyme inhibitor; Ang II, angiotensin II; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ET-1, endothelin-1; ESKD, end-stage kidney disease; FDA, United States Food and Drug Administration; FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; KDIGO, Kidney Disease Improving Global Outcomes; RAAS, renin angiotensin aldosterone system; RaDaR, UK National Registry of Rare Kidney Diseases; RAS, renin-angiotensin system; UK, United Kingdom; UPCR, urine protein-to-creatinine ratio.
References:
1. Abbate M, et al. J Am Soc Nephrol. 2006;17:2974–2984. 2. Lai K, et al. Nat Rev Dis Primers. 2016;2:16001. 3. Wyatt RJ & Julian BA. N Engl J Med. 2013;368:2402–2414. 4. Suzuki H, et al. J Am Soc Nephrol. 2011;22:1795–1803 5. Kohan DE & Barton JM. Kidney Int. 2014;86:896–904. 6. Komers R & Plotkin H. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877–R884. 7. Raina R, et al. Kidney Dis. 2020;6:22–34. 8. Reich HN, et al. J Am Soc Nephrol. 2007;18:3177–3183. 9. Pitcher D, et al. Clin J Am Soc Nephrol. 2023;18(6):727–738. 10. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. Kidney Int. 2021;100(4S):S1–S276. 11. Barbour SJ, et al. JAMA Intern Med. 2019;E1–E11. 12. QxMD IgAN prediction tool for adults at biopsy. Accessed November 2022. https://qxmd.com/calculate/calculator_499/international-igan-prediction-tool-at-biopsy-adults. 13. Barbour SJ, et al. Kidney Int. 2022;102:160–172. 14. QxMD IgAN prediction tool for adults post-biopsy. Accessed November 2022. https://qxmd.com/calculate/calculator_839/international-igan-prediction-toolpostbiopsy-adults
You are about to leave LowerProteinuria.com
Click Continue to leave this site. Click Cancel to remain on LowerProteinuria.com
Cancel